
Green Gas has become a staple airsoft consumable for powering GBBs. At US$15 a can, gas is the biggest running expense in GBB use. I'm a GBB enthusiast! I play twin PKIIs with extended 52rnd mags akimbo style against AEGs sometimes. I played my GBBs so much that I contrived ways to power my GBBs with wierd gas like airbrush propellants.
I first grew suspicious when I realised that many brands of “Green” gas were quite flammable. I questioned: "If green gas was supposed to be a refrigerant; why would it be so flammable?” It turns out that propane is used by the refrigeration industry as a refrigerant. They're just not allowed to use it to aircondition moving things like automobiles in case of a crash.
I decided to investigate further and found the following things about many brands of “green” gas:
- They're very flammable.
- They exert the same pressure as a torch tank of propane at three temperatures (freezer, refrigerator, room temp).
- They have the same density of propane at 1atm and at room temperature.
- The molecule emblazoned on gas cans (CH2FCF3CH3) is fictitious: carbon does not have enough available. electron valences to hang onto that many hydrogen and fluorine atoms (remember high school chemistry?). It also does not appear on any lists of refrigerants currently used.
- An analysis done at an analytical laboratory with a gas chromatograph/mass spectrometer indicates that the primary component of 3 types of green gas is in fact propane!
Well that's five nails in that coffin now.
The good news is that we have many years of safe propane history. The use of GBBs at indoor fields has not shown to cause fires, and propane does not appear to degrade most airsoft elastomers and plastics.
FPS Comparison
I have some chrony results comparing propane from a Coleman stove tank and a brand of gas called "Shooting Air". Tests were conducted with a SVI 3.9" with metal slide shooting 0.25g bbs. Shots were done with a completely filled mag allowed to warm up to room temperature for 30min. Shots were fired every 5 seconds to reduce cooldown.
| Propane (fps) | 289 | 285 | 283 | 275 | 273 |
| Shooting Air (fps) | 281 | 282 | 281 | 277 | 280 |
Flammability
Well, this one's easy. I drew 60ml of propane into a very large syringe and blew it into a lighter flame. The flame properties of green gas are identical to the flame properties of propane drawn from a torch tank.
Several video clips are floating around of airsofters igniting the gas from their GBBs. Although it looks cool, I strongly warn against this practice. Many of us have had some sort of GBB malfunction at some point in time which results in the mag valve locking open and the magazine dumping it's remaining gas charge in one burst. This could result in an unexpected gout of fire out of the various ports of your GBB with very painful results.
Gas Chromatography/Mass Spectrometer (GC-MS) Analysis
It's hard to explain this one succinctly. A short explanation would state that I contracted an analytical lab with big expensive equipment who told me that the gas samples I provided them were propane. The tests were done at Analest Laboratory at the University of Toronto on a Perkin Elmer AutoSystem XL gas chromatographer with a mass spectrometer detector. Jet was the first gas tested, and showed to be propane. Subsequent tests were expensive ($120), and I opted to blend “Shooting Air” and “Green Power” branded gas, and all the mass spectroscopy readouts were comparable to propane library data. I then got them to run a sample drawn from a Coleman propane tank for comparison, and the results were virtually identical to the green gas samples.
Here are some scans of reports provided to me by the lab:


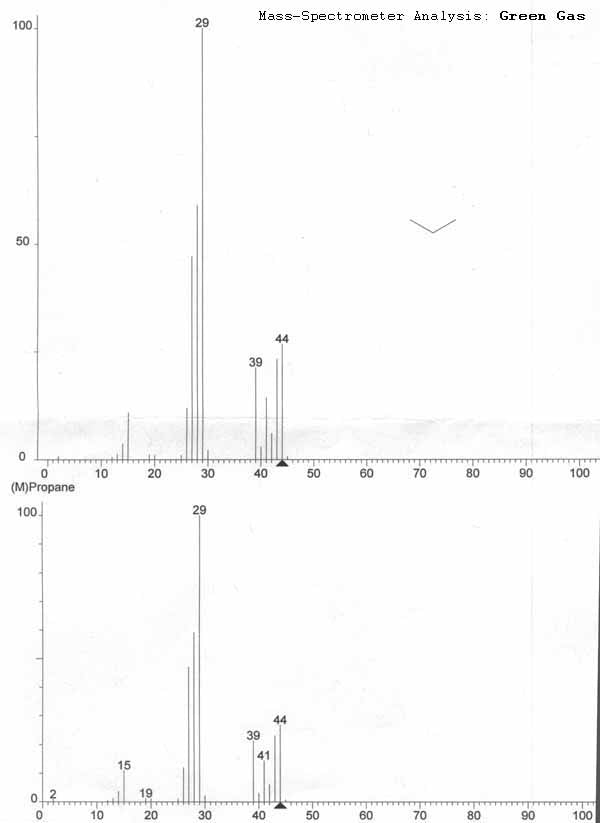



One can conclude that the 3 brands of green gas tested are propane. The three brands of green gas provide very similar results to the test on propane from a Coleman camp stove bottle. For those who would like a better understanding of the laboratory tests conducted, read on.
GC/MS Crash Course
Please realize that my description of spectroscopy is very simplified. Many students have devoted post graduate degrees in the applications of spectroscopy so it's hard to communicate a complete understanding of this field especially since I have little direct experienced with this class of equipment.
The system used was a Perkin Elmer AutoSystem XL Gas Chromatograph with a mass spectrometer detector (GC-MS).
Gas chromatography (GC) is a technique for separating gaseous components of a sample. A gas sample is injected into a coil of tubing with a very special internal coating which has a varying affinity for different molecules. The tubing is coiled to save space and is often referred to as a GC column. At the beginning of the test, sample gas is blown through the tubing and the molecules adsorb to the coating for a period of time. The coil is gradually warmed and the adsorbed molecules gradually release from the column at different times depending on their affinity for the column coating.
Various columns are made to adsorb different classes of molecules. PE manufactures a variety of columns with adsorbance behaviors calibrated against known gases i.e. a sample of known composition can be run on a GC machine and it's output observed for calibration purposes.
A detection device senses the release of a molecule (pure GC devices have a component similar to a Geiger particle counter) and it's time of detection is recorded. Since different molecules have different affinities for the coating in the GC column, the time of release can be used to determine what molecules were present in a gas sample.
The mass spectrometer (MS) portion of the instrument further separates particles from the GC by sorting them by mass. This provides further confirmation of information provided by the GC process. Combining the time of release from the GC process and the mass of individual particles provides a statistical count of various mass releases at various times. Plotting this data provides a signature which can be compared against a library of known compounds i.e. the MS population plot is an indication of sample composition. Data signatures can be compared against a library of known signatures to determine sample components.
Final Analysis
In the case of my analysis for green gas, two major peaks can be seen. The leftmost peak is a peak coinciding with air trapped in the instruments sampling equipment. This peak is present in all 3 tests ("Jet", "Shooting Air/Green Power", Coleman stove propane). There's still only one other major peak which coincides with where the Coleman propane peak lands. Since the green gases only have peaks coinciding with the Coleman propane, all three brands are primarily propane.
In my first test, Jet gas was tested separately with a generic column. I was trying to confirm that it was propane so I had it's test results compared against library data instead of testing it against propane. The second and third tests I commissioned used a light gas analysis column which was optimal for propane assays.
Conclusions
I can say with very high confidence that the contents of three brands of green gas are propane. Although there are many brands of green gas are available, any that are flammable are propane. At the very least any green gas which happens to be flammable cannot be R22 (green gas is often claimed to be R22) is not flammable ( ).
Furthermore, current green gas cans (impact extruded aluminum without overpressure relief feature) cannot safely store propane. These green gas cans do not meet the US Department of Transportation (DOT) or Transport Canada's (TC) requirements for safe storage of propane. Disposable propane containers are required to withstand content pressures exerted at 55C/131F without bursting - Arnie's Airsoft reported on one unfortunate UK player who had a can explode on the roof of his car, with fragments puncturing the vehicle.
Legitimate propane cans are recognized by DOT/TC bodies, and are proven, safe containers for our GBB gas.